M3339燃料電池(TELEDYNE) T1-B-2C
直線:010-59410801(吳小姐)
手機:15330289801 /15330289808/(吳小姐)
18910282265/15330289777(吳小姐)
QQ:1462663658
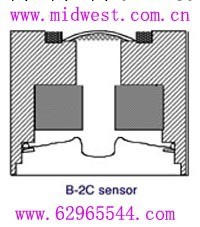
The T1-B-2C trace oxygen sensor is based on an electrochemical system with a silver-plated electrode as the cathode, lead as the anode (pressed granular lead), and an aqueous solution of 10% potassium hydroxide as electrolyte.
The following drawing shows a cross-sectional view of the sensor. In the presence of oxygen, the sensor creates a current that flows from cathode to the anode as a result of the following electro-chemical reactions:
Cathode: 02 + 2H20 + 4e- -----> 4OH-
Anode: 2Pb --> 2Pb2+ +4e-
A standard T1-B-2C has 6 grams of lead in solid form when the cell is made. After the cell has been used, the solid lead is slightly reduced and the ionic lead inside the electrolyte increased. The ionic form of lead in 10% KOH is at the ppm level. The life of this sensor will depend on the history of the sensor, such as how long it has being exposure to high oxygen levels and / or how long it has been used.
Since the T1-B-2C is design for sensing ppm levels of oxygen, the lead consumption is very low. Most of the lead is not consumed and only a small amount is used by the cell during the lifetime of the sensor. Therefore, at end of the T1-B-2C's sensor life, the lead concentration in the electrolyte solution is at a few thousands ppm level and majority of the lead is still in solid form.
Sometimes, lead oxide is formed on the anode (note: lead oxide is also in solid form, reddish crystals over the lead). The current generated by the sensor at ppm levels is so low that most of the lead at the end of life is still in solid form. More importantly, at the end of life, the limiting factor is water loss from the electrolyte and not lead consumption.
T1-B-2C是一密封的電化學裝置,不需要清洗電療、添加電解液等維護。該傳感器隻對氧有響應,所以可準確測量幾乎100%的碳氫化合物中的氧。

批發市場僅提供代購諮詢服務,商品內容為廠商自行維護,若有發現不實、不合適或不正確內容,再請告知我們,查實即會請廠商修改或立即下架,謝謝。